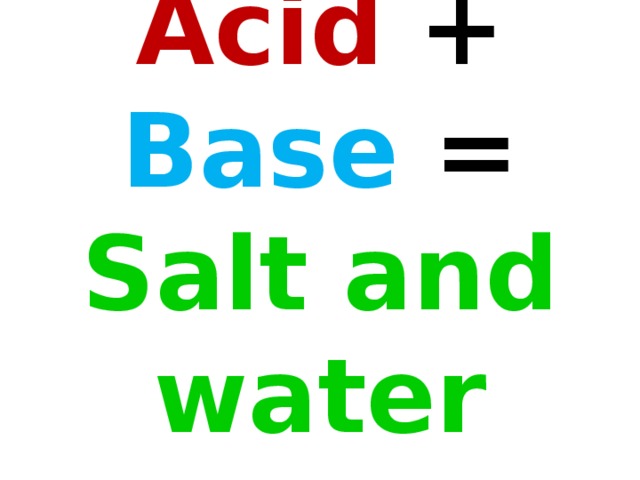
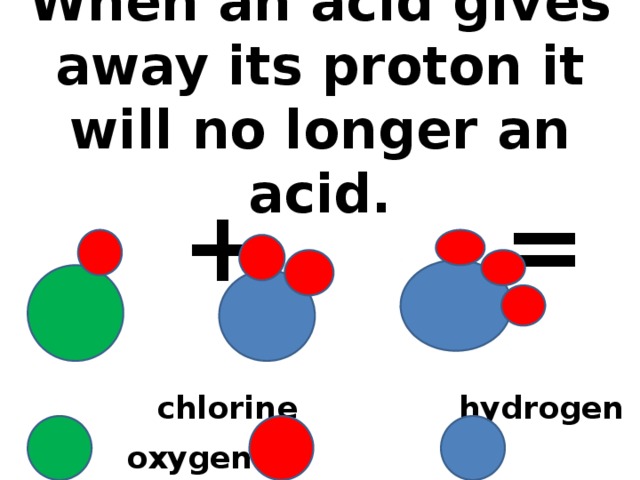
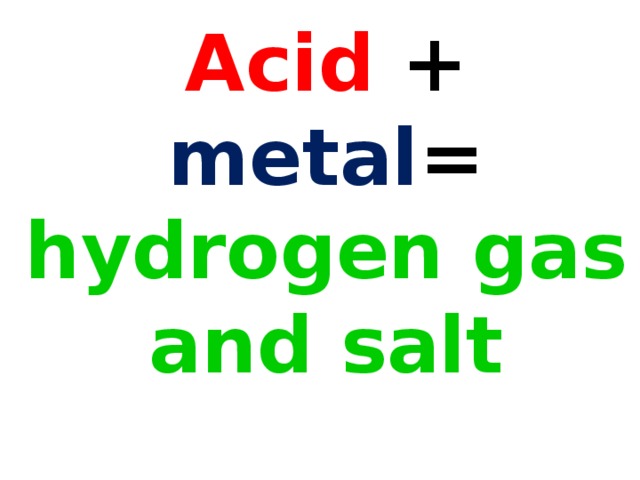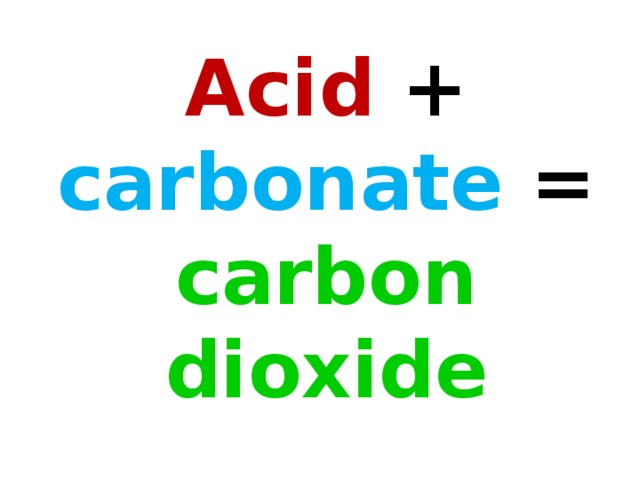Обучение основным понятиям кислоты и их взаимодействие с другими веществами.
Создайте Ваш сайт учителя Видеоуроки Олимпиады Вебинары для учителей
"Понятие кислот и их взаимодействие с другими веществами"
Вы уже знаете о суперспособностях современного учителя?
Тратить минимум сил на подготовку и проведение уроков.
Быстро и объективно проверять знания учащихся.
Сделать изучение нового материала максимально понятным.
Избавить себя от подбора заданий и их проверки после уроков.
Наладить дисциплину на своих уроках.
Получить возможность работать творчески.
Просмотр содержимого документа
«Acids»
Просмотр содержимого презентации
«acids»
Полезное для учителя
Распродажа видеоуроков!
1860 руб.
2660 руб.
1880 руб.
2690 руб.
1970 руб.
2820 руб.
1760 руб.
2510 руб.
ПОЛУЧИТЕ СВИДЕТЕЛЬСТВО МГНОВЕННО
* Свидетельство о публикации выдается БЕСПЛАТНО, СРАЗУ же после добавления Вами Вашей работы на сайт
Удобный поиск материалов для учителей
Проверка свидетельства