презентация по теме "Уравнение состояния идеального газа" на английском языке
Создайте Ваш сайт учителя Видеоуроки Олимпиады Вебинары для учителей
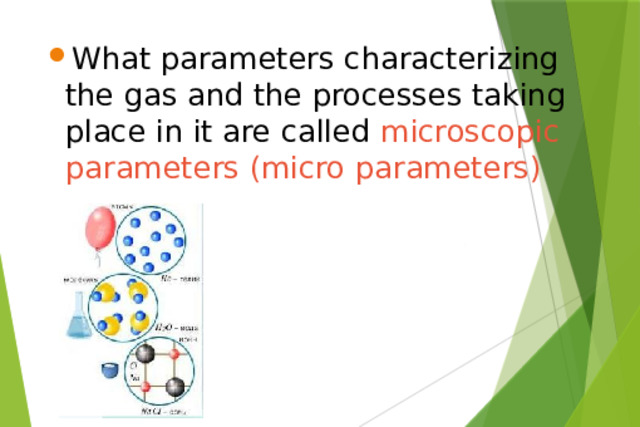
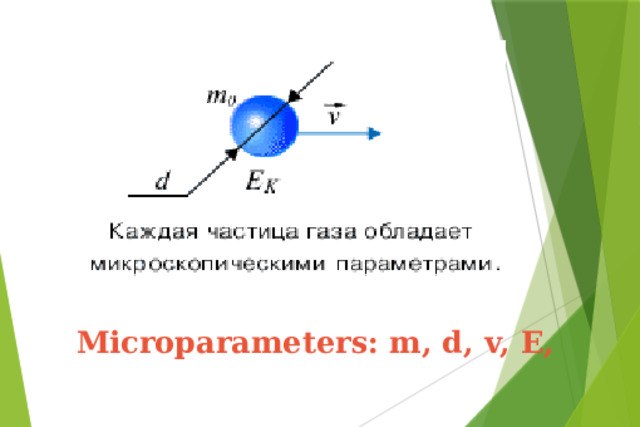
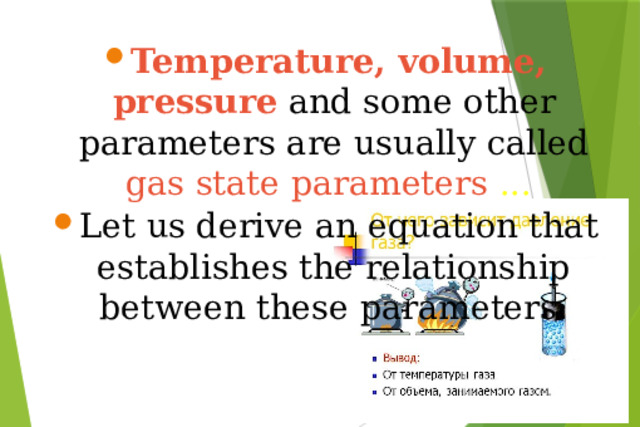
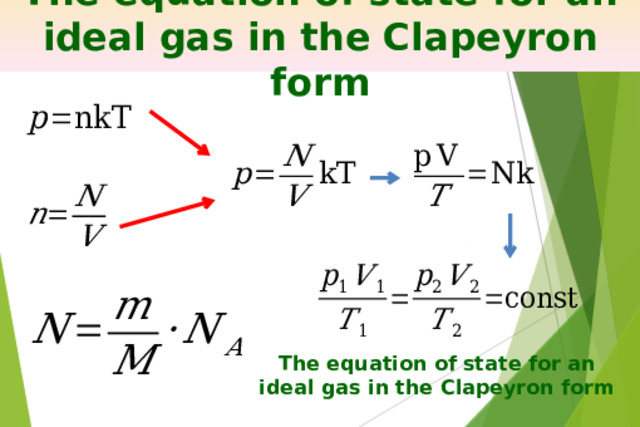
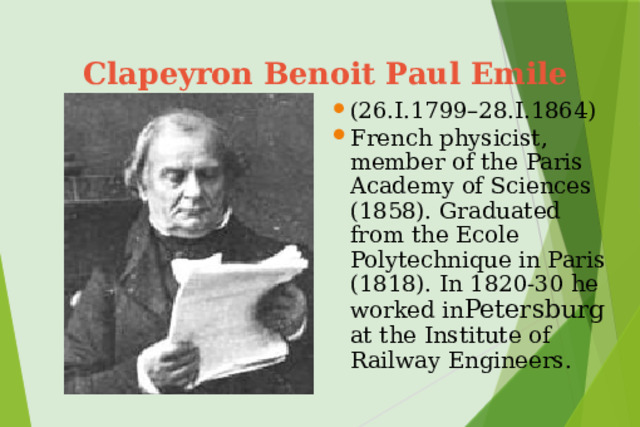
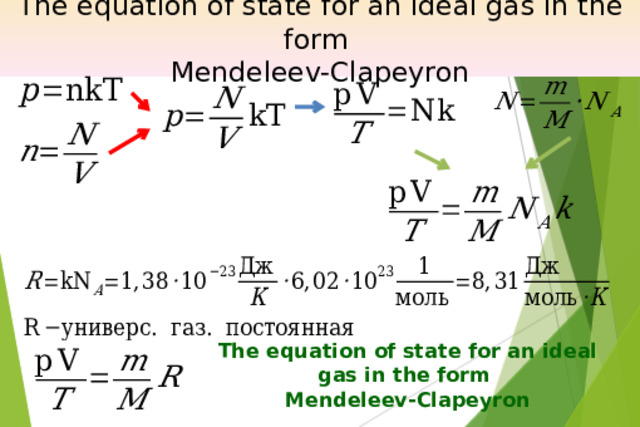
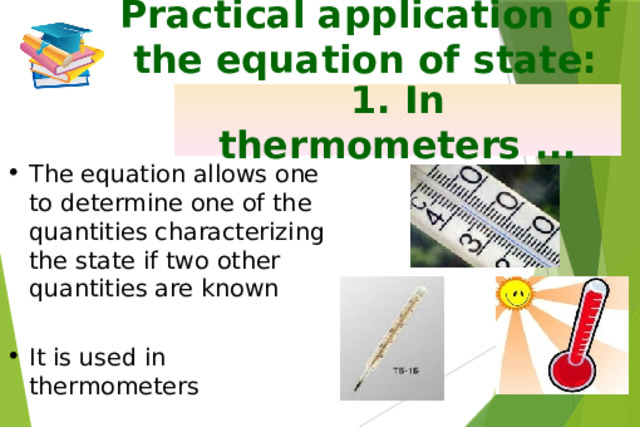
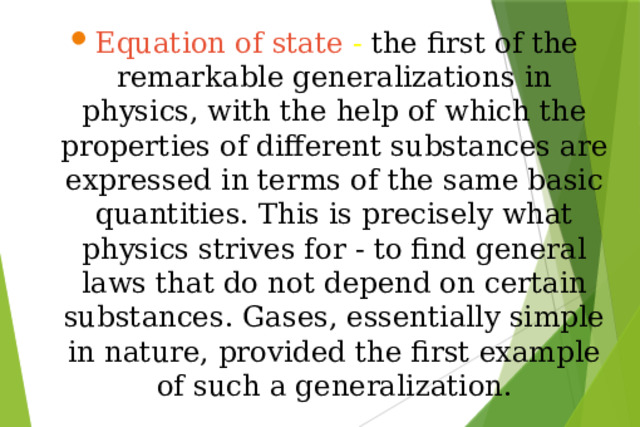
Презентация на урок по физике Ideal gas equation of state
Вы уже знаете о суперспособностях современного учителя?
Тратить минимум сил на подготовку и проведение уроков.
Быстро и объективно проверять знания учащихся.
Сделать изучение нового материала максимально понятным.
Избавить себя от подбора заданий и их проверки после уроков.
Наладить дисциплину на своих уроках.
Получить возможность работать творчески.
Просмотр содержимого документа
«Презентация на урок по физике Ideal gas equation of state»
Полезное для учителя
Распродажа видеоуроков!
1880 руб.
2690 руб.
2040 руб.
2920 руб.
1880 руб.
2690 руб.
1830 руб.
2620 руб.
ПОЛУЧИТЕ СВИДЕТЕЛЬСТВО МГНОВЕННО
* Свидетельство о публикации выдается БЕСПЛАТНО, СРАЗУ же после добавления Вами Вашей работы на сайт
Удобный поиск материалов для учителей
Проверка свидетельства